Excess Reactant
Web An excess reactant is the reactant that is present in excess in a reaction mixture. The limiting reactant or limiting reagent is the first reactant to get used up in a chemical reaction.

Excess Reactant Easy Science Chemical Reactions Easy Science Excess
It shows you how to perform stoichiometric calculations an.

. Web In such instances there are no limiting or excess reagents. It is also known as an excess reagent. Web This chemistry video tutorial explains how to find the amount of excess reactant that is left over after the reaction is complete.
The molar mass of Na 23 grams per mole. Web In order to find the limiting reagents excess reagents and products in this reaction you need to do the following. The percentage excess is calculated using the amount of excess material relative to the quantity.
Web - What is an excess reactantSometimes you may encounter limiting reag. Known Amount of Limiting Reactant. This is financially efficient.
Web An excess reactant is a reactant present in an amount in excess of that required to combine with all of the limiting reactant. We can observe the presence of excess reactant at the beginning of the reaction at the progression and at the end as well. This indicates 23 grams of sodium will react with 71 grams of chlorine.
2 x 42394 g LiCl 1 x 37996 g F2 Calculated Amount of Excess Reactant. 318 g LiCl - 212 g LiCl 106 g LiCl. 212 g LiCl Leftover Excess Reactant.
The same process should go for the products. This is explained through the following example. Excess reagent is the reactant that is present in excess in a.
Now the mass of the excess reactant that is consumed in the reaction and the mass that is unreacted should be measured respectively. Determine the limiting reagent if 100 g of each reagent are present at the beginning of the reaction. Identify the excess reagent as well as how many grams of the excess reagent will remain when the.
Web Introduction to Limiting Reactant and Excess Reactant. Web Excess Reactant Needed. It follows that an excess reactant is one remaining in the reaction mixture once all the limiting reactant is consumed.
- What is a limiting reactant. Therefore after the completion of the reaction some amount of this reactant still remains since it is in excess. Web The reactant left over when the reaction is complete is the excess reactant.
Web The reactant forming larger amount of product is the excess reagent. Web Then subtract the number of moles of excess reactant used from the total moles of excess reactant available to find the actual amount of excess moles. Web The EXCESS REACTANT is the substance that is excess because of the limiting reactant and the reactantreagent that gives the excess amount in a reaction.
Chemical Reaction occurs in a perfect stoichiometry among reactants and products. Web This chemistry video tutorial shows you how to identify the limiting reagent and excess reactant. You need to start with th.
In this video we are going to answer these questions. Web Balance the chemical equation 2 N a C l 2 2 N a C l. To solve the problem you must identify which of the reactant is going to run out.
Web What is an Excess Reactant. Those are called the excess reactants. An excess reactant is a substance that is not wholly consumed or entirely reacted in a chemical reaction.
Which one is the excess reactant when 80 g of Na 2 O 2 combines with 30 g of water. Limiting reagent is the reactant of a particular chemical reaction that limits the formation of the product. A good way to ensure that one reactant fully reacts is to use an excess of the other reactant.
Once the limiting reactant gets used up the reaction has to stop and cannot continue and there is extra of the other reactants left over. The amount of product formed is independent of the quantity of excess reactant. Difference Between Limiting Reagent and Excess Reagent Definition.
To determine which of the reactants is the limiting one choose one then use stoichiometric ratios to calculate how. 950 g F2 Mass Ratio. It is governed by the other reactant which is completely used and can therefore cannot react.
Molar mass of Cl2 2 355 71 grams per mole. To find the excess reagents we need to know their molar masses. Web An excess reactant is the reactant left over after the reaction stops when the limiting reactant is completely consumed.
Within chemical reactions there is always a limiting reactant and an excess. The COMPLETION is when there will be product formation. Web The reactant in excess also known as the excess reagent is the amount of chemical remaining after a completed reaction.
For example if the ratio is 12 and there are 05 moles of reactant A 1 mole of reactant B will be used and 1 mole of B will be in excess.

Introduction To Limiting Reactant And Excess Reactant Chemistry Help Apologia Chemistry High School Chemistry

Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube Ch 8 Teaching Chemistry Chemistry Notes Chemistry
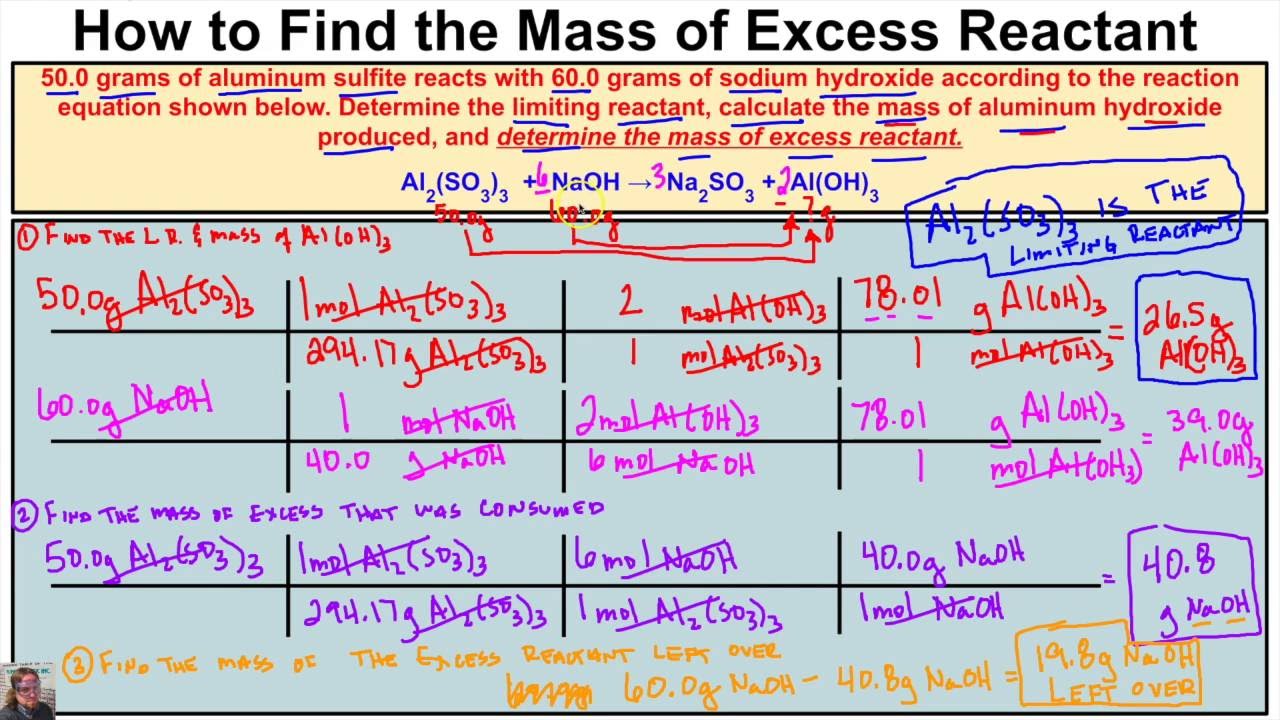
How To Calculate The Mass Of Excess Reactant Left Over In A Chemical Rea Chemistry Class Chemical Reactions Ap Chem

Introduction To Limiting Reactant And Excess Reactant Science Sciencewithtylerdewitt Tylerdewitt Tu Chemistry Help Apologia Chemistry High School Chemistry
Comments
Post a Comment